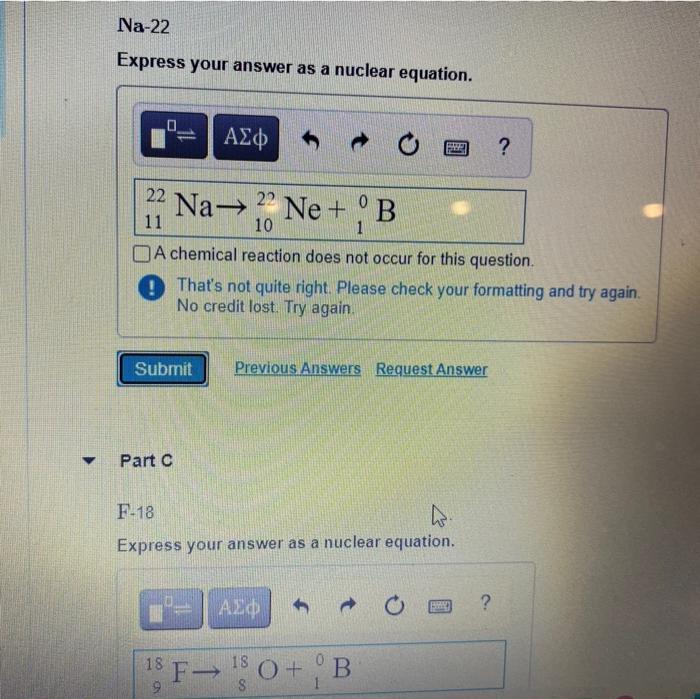
Enter a Nuclear Equation for Positron Emission by Each Nuclide
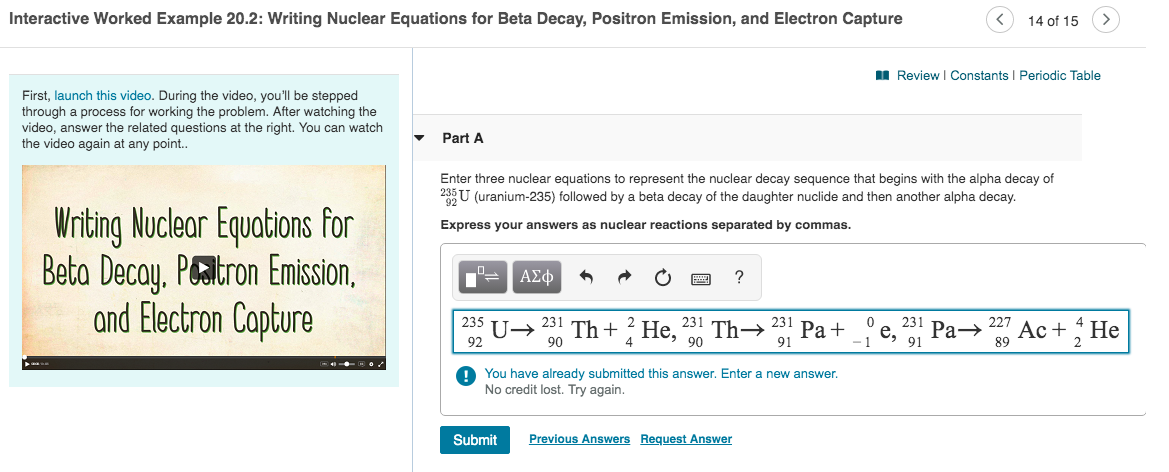
Part A Enter three nuclear equations to represent the nuclear decay sequence that begins with the alpha decay of U-235 followed by a beta decay of the daughter nuclide and then another alpha decay Express your answers as nuclear reactions separated by commas. 1 on a question.
Solved Enter A Nuclear Equation For Positron Emission By Chegg Com
A operatorname co-55 b Na-22 c mathrm F-18.
. This question asks us to complete the nuclear equations written here for the Alfa decay processes off four different isotopes so we can go through them on my one. Transcribed image text. You may want to reference Pages 619-622 Section 175 while completing this problem.
For the next one we have nitrogen 13. WU-Th 4He Th Pa_98 Pa Ac He Previous. The nuclide nitrogen-18 undergoes beta emission.
Write three nuclear equations to represent the nuclear decay sequence that begins with the alpha decay of U-235 followed by a beta decay of the daughter nuclide and then another alpha decay Part C Write the third nuclear equation from this sequence. 234 0 Z so Z must be 234. Arrow_forward Write the balanced nuclear equation for the induced transmutation ofaluminum-27 into sodium-24 by neutron bombardment.
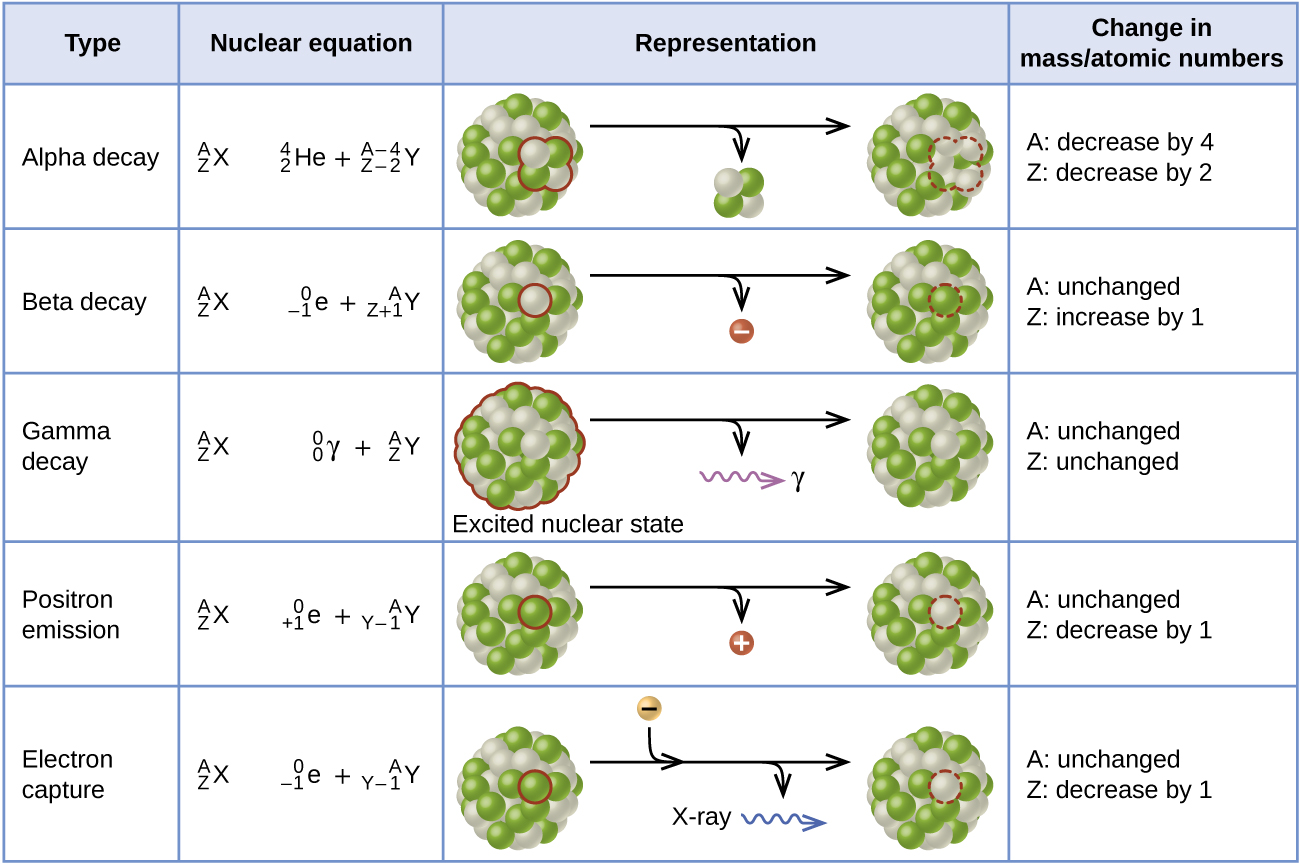
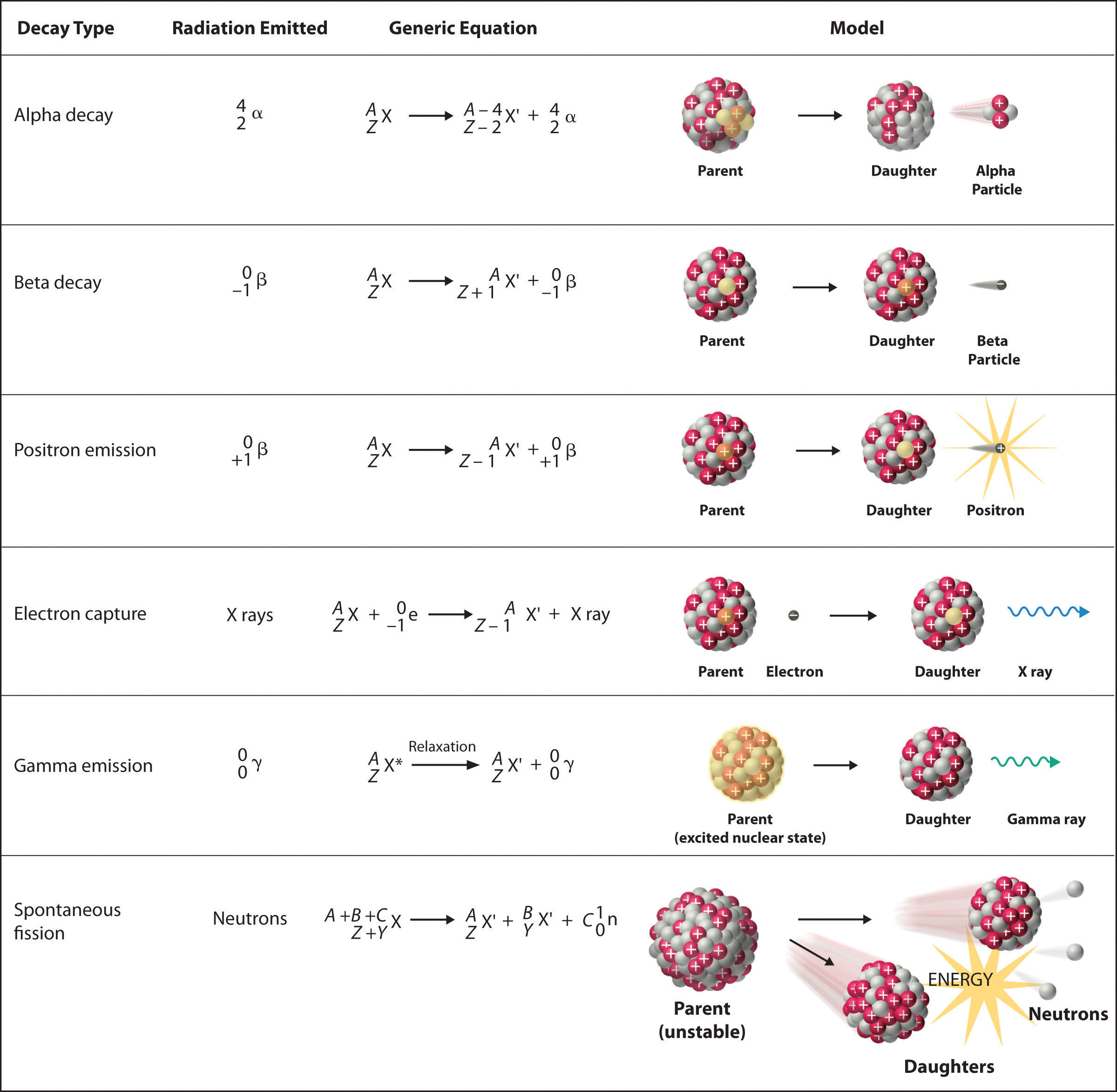
Show the atomic number and mass of each species in the equation. Left numbers must add up on both side. The spontaneous and random emission of particles or electromagnetic rays in nuclear decay of a sample.
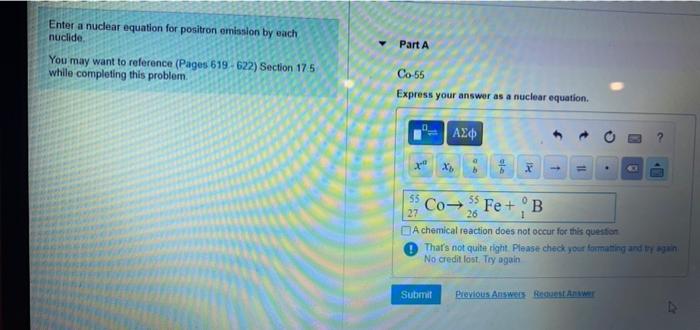
Write a balanced nuclear chemical equation that describes this process. So 90 -1 A and A must be 90 1 91. Going to a positron.
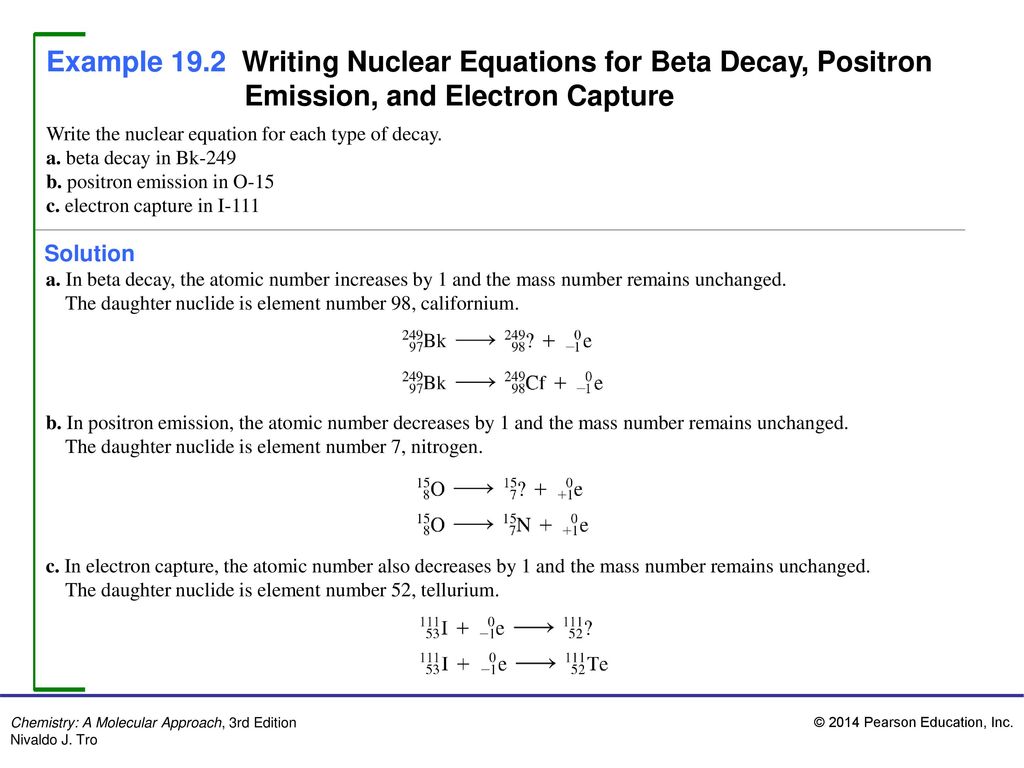
Part A Enter a nuclear equation for positron emission by each nuclide. SOLVEDWrite a nuclear equation for positron emission by each nuclide. Part D Write the nuclear equation for the positron emission of Al-26 Part E Write the.
So six plus one gives us the original seven. You may want to reference Pages 619 - 622 Section 175 while completing this problem. And atomic number five is boron.
18 uh and this has an atomic number 84 it can undergo Alfa decay. 1 Answer to Write a nuclear equation for the indicated decay of each nuclide. Potassium 40 and xenon 118 40 19 K -- 01 4018 Ar 11854 Xe -.
90Th234 -1e0 AXZ. Write the complete nuclear equation for the bombardment of a 27 Al with an alpha particle to yield 30 P. Write a balanced nuclear equation for the following.
Were being asked to write the nuclear equations for a variety of different um decay Uh situations. Solution for Write a balanced nuclear equation for the following. The first reaction shows polonium 2.
Fill in the blanks in the partial decay series. O NUCLEAR CHEMISTRY Brandon V Writing the equation for a typical radioactive decay The potassium-38 nuclide radioactively decays by positron emission. N-13 positron emission e.
Express your answers as nuclear reactions separated by commas. This is a modal window. This then allows the atomic number of the are the mass number of the product to be 13 and the atomic number is going to be six.
Part a enter three nuclear equations to represent the nuclear decay sequence that begins with the alpha decay of 235 92u uranium-235 followed by a beta decay of the daughter nuclide and then another alpha decay. The 1st 1 were being asked to write out the equation for you to 34 undergoing Alf. A radioactive particle that is emitted from the nucleus and decreases the atomic number by one.
Cr-51 electron capture word_media_image1png. Video Player is loading. The element which has atomic number 91 is Pa so the full equation is.
Part b enter the nuclear equation for the positron. Beta is an electron which is -1e0. Write the balance nuclear equation for the positron decay of each of the following radioactive isotopes.
Up to 256 cash back Write a nuclear equation for the indicated decay of each nuclide. Right numbers must add up on both sides. Is a process in which two nuclei or nuclear particles collide to produce different products than the initial particles.
The study of changes in the nucleus of an atom. Beginning of dialog window. The nuclide phosphorus-28 undergoes positron emission.
Then we have oxygen 15 undergoing positron emission and we get a mass number of 15. So for Th we write 90Th234 beta. Enter a nuclear equation for positron emission by each nuclide.
Co-55 Express your answer as a nuclear equation. Answers Correct The radioactive. You may want to reference Pages 619 - 622 Section 175 while completing this problem.

16 1 Nuclear Equations Chemistry Libretexts
Solved I M Quite Sure That This Answer Is Correct But Don T Chegg Com

Radioactive Decay Chem 1305 General Chemistry I Lecture
Solved Enter A Nuclear Equation For Positron Emission By Chegg Com

Solved Write A Nuclear Equation For Positron Emission By Chegg Com
21 3 Radioactive Decay Chemistry

Solved Nuclear Reactions Alpha Emission Nuclear Decay Chegg Com
Example 19 1 Writing Nuclear Equations For Alpha Decay Ppt Video Online Download

Writing Beta Decay Nuclear Equations Youtube

Solved 2 Point Scandium Sc Decays By Emitting A Positron What Is The Nuclide That Is The Product 21 Of The Decay 43 A Sc 21 44 B Ca 20 43 C Ca

Solved 4 Write A Nuclear Equation For The A Positron Chegg Com
21 2 Nuclear Reactions Chemistry Libretexts
Solved Enter A Nuclear Equation For Positron Emission By Chegg Com

Chemistry 1412 Quiz 21 Flashcards Quizlet
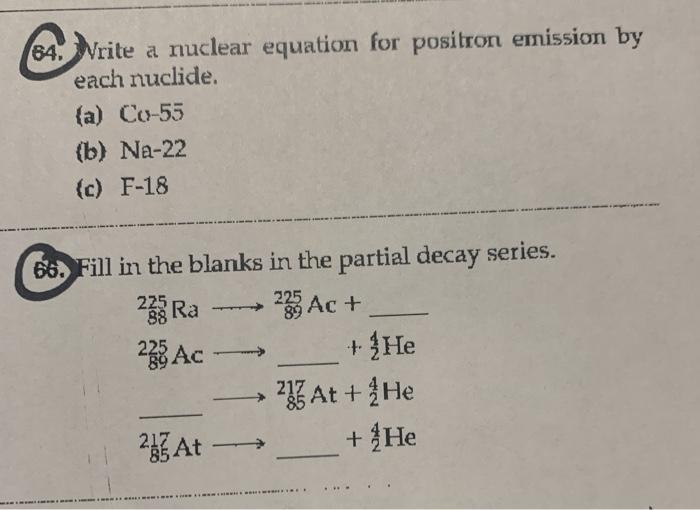
Solved 64 Write A Nuclear Equation For Positron Emission By Chegg Com
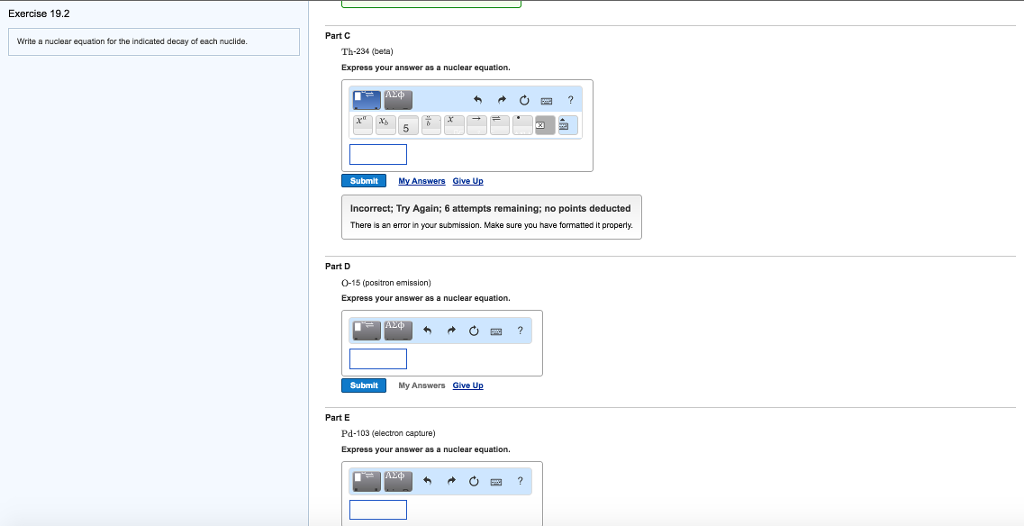
Solved Write A Nuclear Equation For The Indicated Decay Of Chegg Com

Types Of Radioactive Decay Nuclear Stability
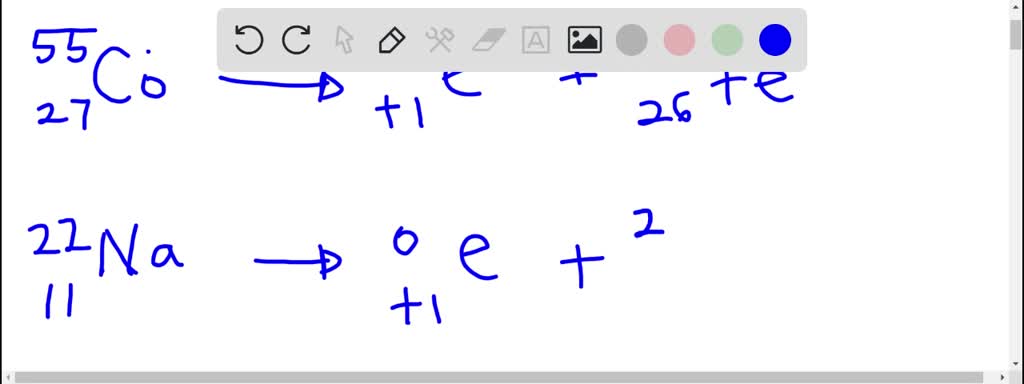
Solved Write A Nuclear Equation For Positron Emission By Each Nuclide A Co 55 B Na 22 C F 18
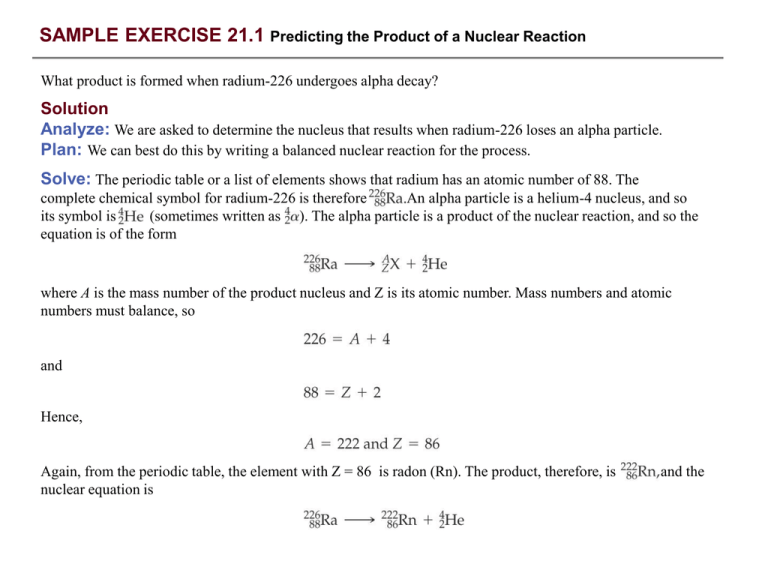
Comments
Post a Comment